INTRODUCTION
Cell therapies show significant promise to enhance human health through the administration of viable cells into a patient’s body. During the manufacture of these often life-saving therapies, maintaining cell viability is a critical concern, as non-viable cells do not demonstrate the desired therapeutic effect and do not contribute positively to the potency of a dose. Therefore, cell viability must be closely considered when choosing process conditions and equipment for each step of the cell therapy manufacturing process.
Two common process steps, cell harvest and cell washing, can significantly reduce cell viability if cells are exposed to high shear forces. During these steps, the efficiency of cell recovery is also of critical concern, as cells which escape to waste-streams will not be recovered. In this case study, we present data demonstrating the performance of a single-use centrifuge (CARR Biosystem’s UniFuge® family) for the harvest of cells in cell therapy production. Specifically, the key performance indicators of cell viability and cell recovery are presented for a variety of common cell types and over a range of processing conditions, such as feed flow rate and g-force.
THE CHALLENGE
While it is critical for cell therapy manufacturers to implement solutions that lead to high viability and recovery rates during cell harvest, many available technologies present a trade-off between the two or fall short on other critical performance requirements. Technologies which present superior separation efficiency (recovery) are often unable to maintain sufficient cell viability, while those technologies which maintain high levels of viability often demonstrate low recovery. Further, many technologies which may perform acceptably in both cell viability and recovery cannot scale to levels required for allogeneic therapies. Finally, cell recovery and viability are highly dependent on the cell type and surrounding environment; therefore, cell harvest process conditions must be optimized through small-scale trials.
THE SOLUTION
As the pioneer in single-use and tubular bowl centrifuge technology, the scalable UniFuge family of cell harvesting systems are ideal for shear-sensitive cells. To obtain optimal efficiency with reliable, repeatable results from applications spanning early-stage discovery to large scale manufacturing, the CARR Biosystems team works closely with customers to optimize processes through detailed demonstrations, followed by world class post-sale support.
THE RESULTS
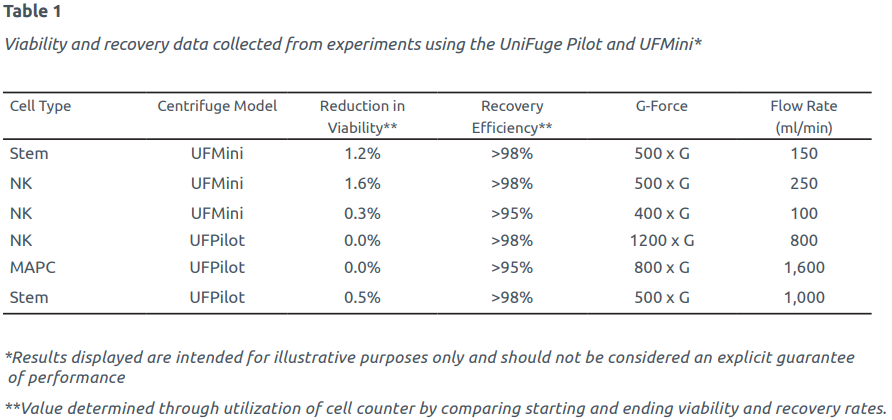
Data collected from numerous studies has proven the UniFuge has minimal impact on cell viability and provides high recovery rates across a variety of cell lines. Table 1 (see page two) provides a sample of experiments which have been run with the UniFuge® Pilot and UFMini®, demonstrating high performance over a wide range of operating conditions and cell types commonly used in cell therapy. As shown in the table, performance data from the UniFuge Pilot and UFMini across various delicate cell types demonstrates industry-leading results in the categories of cell viability, recovery, and processing speed.
ABOUT THE UNIFUGE FAMILY
The UniFuge family provides low-shear separation, high-recovery performance, and fast processing times in three scalable models: UFMini, UniFuge Pilot, and U2K. With a variety of bowl sizes and flow rates ranging from 29 ml/min to 20 L/min, the UniFuge family of single-use separation systems offer both scalability and process efficiency in an aseptic closed system. The UniFuge Pilot was the industry pioneer in single-use centrifuge separation technologies. In 2021 CARR Biosystems released the UFMini to better support customers with a need for smaller scale processing using single-use technology.
ABOUT CARR BIOSYSTEMS
Founded in 1993 in Medfield, Massachusetts, CARR
Centritech introduced the Powerfuge and continued
to develop advanced separation systems for the
Life Sciences and Specialty Chemical markets with
the Viafuge, Centritech, and UniFuge models.
In 2022, CARR Centritech transitioned to CARR
Biosystems with continued emphasis on developing
high-quality products to meet safety and quality
standards throughout a variety of applications
across the globe.