Abstract
Cell harvest is a critical processing step in the production of monoclonal antibodies and other biologic products. In this study, we evaluate the performance of CARR Biosystems’® UFMini single-use centrifuge in the clarification of CHO-S cells by measuring supernatant turbidity and starting/ ending cell viability. The system was evaluated at a feed flow rate of 165 mL/min with a separation force of 3000g. The supernatant turbidity sample result was 3.01 NTU indicating that the vast majority of the CHO-S cells were collected in the cell concentrate with minimal cell breakthrough into the supernatant. Additionally, the change in viability of -1.00% indicates that the UFMini successfully cleared cellular debris and exhibited low shear stress during centrifugation processing. The CARR Biosystem’s UFMini Single-Use Centrifuge successfully purified and concentrated the CHO-S cell feed while exhibiting low shear stress with minimal impact to cell viability.
The results demonstrate the UFMini can be utilized to optimize clarification processing efficiency by providing a user-friendly single-use centrifugation process that maintains cell viability and produces a highly clarified effluent that can help to reduce burden on downstream processes.
Background
Increasing demand for biopharmaceutical drugs has driven innovation in the development and optimization of high-yielding manufacturing processes. With improved media, feed supplements, bioreactor designs, and control of process parameters, Biomanufacturers have achieved multifold increases in cell densities and volumetric productivity from production bioreactors (Sharma, et al, 2017). As a result, the industry has seen a recent increase in Bio-technology Research and Development (R&D), which has applied pressure to developers to deliver clarification technology that allows for an efficient and low shear process that maintains cell viability, produces high product recovery, and can reduce manufacturing costs.
Clarification technology that provides low shear stress on cells is preferred to maintain cell viability, which is critical since non-viable cells can negatively impact overall process efficiency and yields. Additionally, processes which provide closed processing, high flow rates, and high clarification efficiency enable improved production efficiencies and help to ensure product quality.
In recent years, single-use clarification technology has become increasingly popular as the single-use component eliminates the need for Clean In Place (CIP) and Steam In Place (SIP) cycles and decreases processing times, manufacturing costs, and overall footprint.
Materials
- 1 UniFuge® Mini (UFMini)
- 1 UFMini Single-Use Module
- 270 mL of [1x] [.0067M] Phosphate Buffered Saline (PBS)
- Hachman 2100Q Turbidity meter • Vi-Cell Blu Analyzer • 1 L of CHO-S cells
Methods
- Using Albany’s Center for Biopharmaceutical Education and Training (CBET) facilities, materials, and instrumentation, grow CHO-S cells and dilute with [1x] [.0067M] PBS to a final volume of 1 L.
- Collect 200 µL samples in triplicate from the 1 L container of CHO-S cells.
- Prepare the CARR Biosystems UFMini for processing: a. Install the UFMini single-use module and tube-set. b. Program experimental conditions into the UFMini.
- Prefill the UFMini bowl with 270 mL [1x] [.0067M] PBS.
- Feed and centrifuge 1 L of CHO-S cells through the UFMini at 165 mL/min and 3000g: a. Collect 30 mL sample directly from the supernatant stream at the end of the centrifugation run.
- Module discharge of 300 mL of CHO-S cell concentrate. a. Collect 200 µL samples in triplicate from the CHO-S cell concentrate.
- Collect Data: a. Process the CHO-S cell feed, CHO-S cell concentrate, and supernatant samples through the Vi-Cell Blu analyzer. b. Process the post-processing supernatant sample through the Hachman2100Q Turbidity meter. c. Record and analyze results.
Results
Discussion
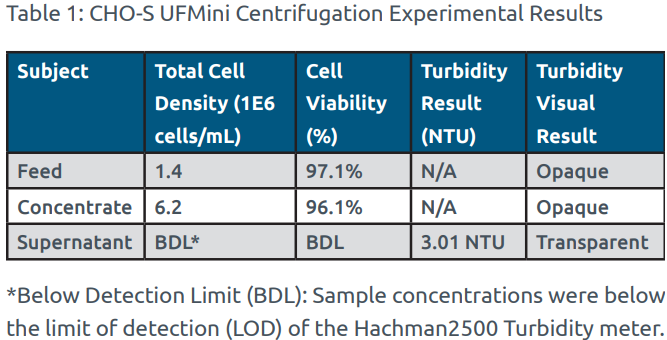
The UFMini provided a simple and efficient clarification process with a <15-minute set-up time and a <30-minute processing time for the 1 L CHO-S cell centrifugation run. Following completion of the centrifugation run, the CHO-S cell concentrate was observed as opaque and the supernatant was observed as transparent, providing visual indication of a successful cell separation process. Post-processing visual inspection was performed to confirm that the UFMini Single-Use module was completely empty following each centrifugation run. As shown in Table 1, the supernatant turbidity sample result of 3.01 NTU provides confirmation that the vast majority of the CHO-S cells were collected in the cell concentrate, and minimal cells passed through into the supernatant during processing. The -1.00% change in viability from the starting CHO-S cell feed to the final CHO-S cell concentrate indicates that the UFMini successfully cleared cellular debris and exhibited low shear stress during processing, which maintained the CHO-S cell viability.
Conclusions
The CARR Biosystem’s UFMini single-use centrifuge successfully purified and concentrated the CHO-S cell feed while exhibiting low shear stress with minimal impact to cell viability. The UFMini single-use tube-set and module allowed for <15-minute set-up time, and eliminated the need for CIP and SIP cycles, which can significantly reduce overall processing times and manufacturing costs. Additionally, the user-friendly Human Machine Interface (HMI), convenient bench-top size, and overall centrifuge design allowed for an efficient and successful cell clarification process.
The results demonstrate the CARR Biosystem’s UFMini allows for optimization of clarification processing by providing an efficient, user-friendly single-use centrifugation process which allows for maintenance of cell viability, improvement of final product yields, and reduction of manufacturing costs and processing times.
Acknowledgments
The CARR Biosystems team is appreciative of the Albany CBET team’s support throughout completion of the experiment.
About the UniFuge® Family
The UniFuge single-use family provides low-shear separation, high-recovery performance, and fast processing times in three scalable models: UFMini, UniFuge Pilot, and U2K®. With a variety of bowl sizes and flow rates ranging from 29 ml/min to 20 L/ min, the UniFuge family of single-use separation systems offer both scalability and process efficiency in an aseptic closed system. The UniFuge Pilot was the industry pioneer in single-use centrifuge separation technologies. In 2021 CARR Biosystems released the UFMini to better support customers with a need for smaller scale processing using single-use technology.
The UFMini is the smallest scale single-use centrifuge in CARR Biosystems’ UniFuge family. The primary applications for the UFMini are in the harvest and washing of cells for use in cell therapy, cellular agriculture, and cell banking — as well as the clarification of cell cultures during production of Monoclonal Antibodies (mAb) and other therapeutic protein products. The UFMini single-use module provides a closed system for sterile processing and eliminates cross-contamination and the need for CIP and SIP cycles, while reducing change-over time between centrifugation runs. Furthermore, the UFMini, which fits on a standard-size laboratory bench, can easily scale up to the larger Unifuge Pilot, or to the even larger manufacturing scale U2k.
References
Sharma, M. K., Raikar, S., Srivastava, S., & Gupta, S. K. (2017). Examining single-use harvest clarification options: A case study comparing depth-filter turbidities and recoveries. Bioprocess Int, 15(2), 40-47.