INTRODUCTION
In the production of cellular-based therapies, media and buffer are often used to remove unwanted proteins, serums, and debris, which then necessitates washing and purifying cells prior to the eventual administration to patients or for research and treatment purposes.1
Although several methods are commonly used to wash cells at the laboratory scale, methods employed in the production of allogeneic cell therapies must meet specific performance requirements including processing capacity, time, aseptic (closed system) operation, and scalability through the therapeutic development process, while maintaining cell viability and preventing the loss of cells into waste streams.
Further benefit can be gained when additional unit operations (e.g., cell harvesting) may be completed efficiently within the same closed system, reducing process complexity, time, and risk. In this case study we present data demonstrating the performance of a single-use centrifuge (CARR Biosystems’ UniFuge® family) in cell washing. Specifically, the key performance indicators of cell viability and washing efficiency are presented when washing cells with common washing solutions used in the manufacturing of cell therapies.
THE CHALLENGE
Manufacturers of cell therapies are operating in a fast growing, yet highly competitive industry, with challenges including high product costs, inadequate reimbursement, and slow market acceptance. To overcome these challenges, manufacturers must focus on optimizing manufacturing processes through the implementation of closed systems as well as efficient and automated processes to translate their discoveries into approved and sustainable therapies.
For those organizations relying on manual cell washing and media exchange processes, the amount of time taken to complete this stage of the separation often takes longer than the initial separation of the cell line itself. Additionally, such processes are often not fully closed which can increase the risk of contamination. In order to decrease the separation and exchange time and to improve safety, a closed-system separation device is needed to process buffer and media solutions while maintaining asepsis and ensuring that cell recovery and viability rates are not jeopardized.
THE SOLUTION
While developing the UniFuge® Pilot and UFMini® separation systems, the CARR Biosystems® team worked closely with customers to develop a solution to offer both buffer flushing and media exchanging features in one closed system. The result was an optimized process for cell washing and media exchanging during cell separation. The process can be adopted to meet the requirements of a specific therapy’s manufacturing process, however the main steps are as follows. During a buffer flush, buffer solutions are flowed through the UniFuge bowl to remove media and debris from the concentrated cell slurry. Subsequent flushes may be performed to increase the washing efficiency. Following the buffer flush(es), media can be introduced into the same bowl and the cells may be re-suspended. Prior to discharging the module’s contents, additional media can be added to the bowl in order to combine media contents for the purposes of reseeding, freezing, or additional exchanges. Upon conclusion of buffer flushing and/or the media exchanging functions, UniFuge customers are able to separate their cell slurries while maintaining aseptic processing within the same system. The individualized approach with buffer flushing and media exchanging allows the customer to maximize cell viability and yield.
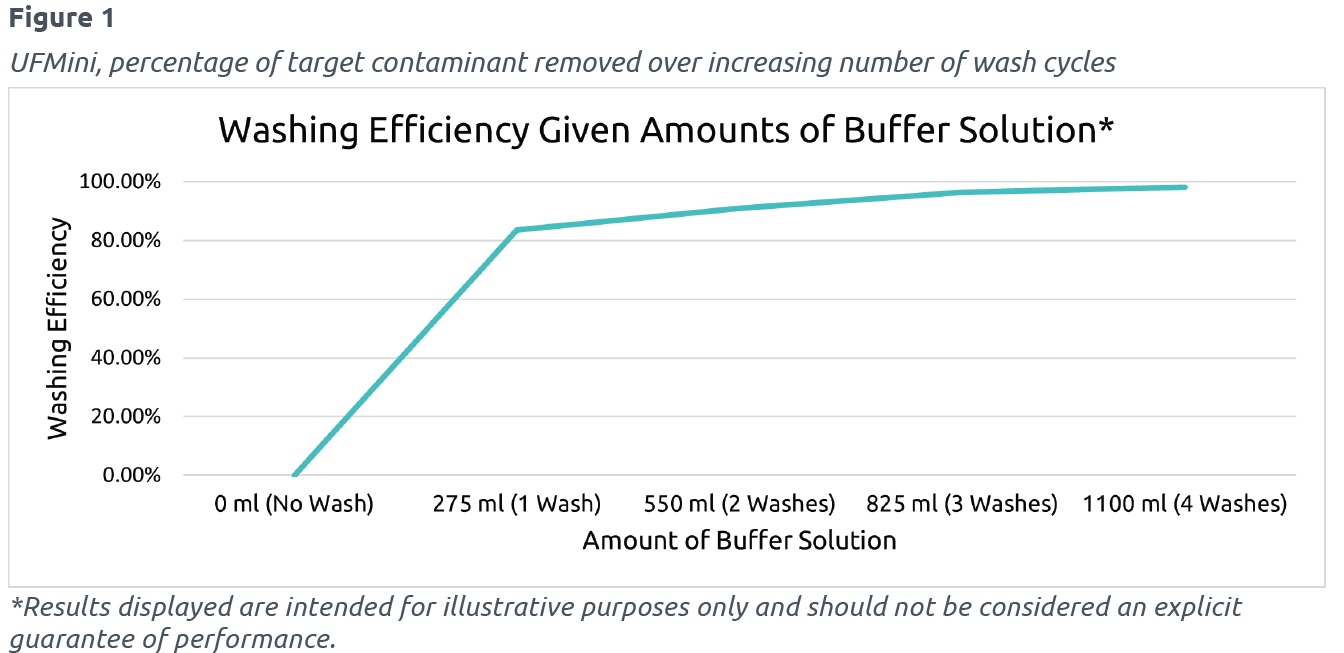
THE RESULTS
a target contaminant removed with increasing numbers of wash cycles. Through the optimization of G-Force and feed rates during the study, greater than 98% of cells were retained through a washing cycle at the optimal condition. This graph provides performance data from the UFMini® highlighting the efficiency of buffer solutions when used with mammalian cells. The performance results of the UFMini with cell washing and media exchanging provides bioprocessing experts the confidence that a single device for cell harvesting and washing is not only possible aseptically but is also an effective method.
REFERENCES
AAT Bioquest. 2019, September 16. Why are cells washed? https://www.aatbio.com/resources/faq-frequently-asked-questions/Why-are-cells-washed