INTRODUCTION
In the production of monoclonal antibodies (mAb), depth filtration has been the predominant technology for primary clarification in single-use processes. By contrast, in traditional bioprocesses based on stainless steel technology, disk stack centrifugation had been the dominant technology.1 Until the advent of single-use centrifugation, there was little choice for the primary clarification step within single-use bioprocesses. With the arrival of the single-use centrifuge, bioprocessing development engineers again have a choice in primary clarification technology.
The UniFuge Pilot® single-use cell harvesting system is specifically designed for primary clarification of mammalian cell cultures (such as CHO cells) and harvesting. This case study presents a cell culture clarification process using the UniFuge Pilot.
THE CHALLENGE
With increasing cell densities and single-use bioreactor volumes up to and beyond 2000L, manufacturers using filtration for primary clarification require a growing number of filters to clarify each batch of product. This presents several challenges which include the large footprint required within the cleanroom and warehouse to store and operate the filtration process, the significant amounts of water required to pre-flush large depth filtration systems prior to use, and the cost of disposal after use. Additionally, the large filtration surface area and hold-up volume increases the risk of protein loss within depth filters.
High shear clarification methods, such as disk stack centrifugation, can also present a challenge to bioprocesses due to the effects of cell lysis on product quality, yield, and downstream purification. The release of host cell proteins, DNA, and specifically enzymes such as lactate dehydrogenase (LDH) from lysed cells are known to negatively impact mAb production.1,2
A technology that reduces filtration footprint while maintaining a low-shear environment for cells is needed.
THE SOLUTION
The UniFuge Pilot meets this need. Its unique design specifically maintains cell viability and allows for efficient separation under lower g-forces than those typically found during separations using a disk stack centrifuge. The gentler, low-shear UniFuge operation results in minimal LDH change during harvest, indicating that cells largely remain in-tact. Additionally, when compared to the operation of depth filters, the UniFuge requires no pre-flushing, thereby reducing water consumption; eliminates the need for filter disposal; and uses less space in the production and warehouse environments.
THE RESULTS
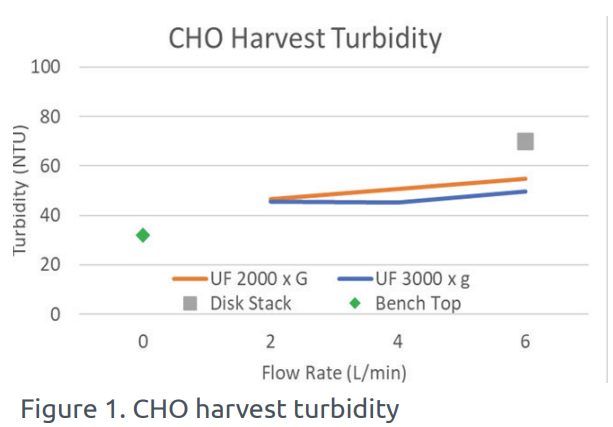
In this study, a 200 liter batch of CHO cell culture was processed under varying parameters. The UniFuge Pilot showed improved turbidity (NTU) compared to the traditional disk stack process. The performance of UniFuge Pilot, the disk stack, and a baseline case reference of a manual bench-top centrifuge are shown in Figure 1 below.
The amount of LDH was also measured during the harvest using the UniFuge Pilot and compared to the baseline case reference. A minimal increase in LDH was detected using the UniFuge Pilot, indicating high cell viability through low-shear operation.
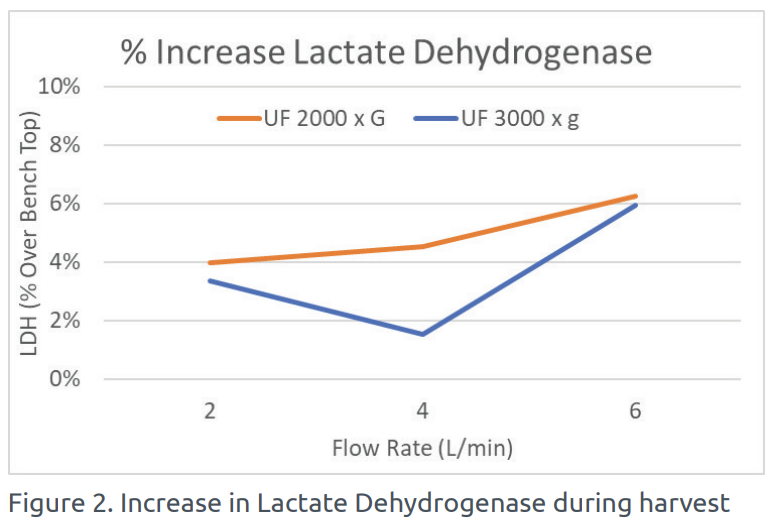
DISCUSSION
This case study has shown that the UniFuge Pilot significantly reduces turbidity levels of CHO culture, while maintaining low levels of LDH. This efficient and low-shear clarification can provide significant advantages in processing efficiency over other harvest methods including depth filtration and disk stack centrifugation.
REFERENCES
Shukla, A.A. and Hinckley, P. (2008), Host cell protein clearance during protein a chromatography: Development of an improved column wash step. Biotechnol Progress, 24: 1115-1121. https://doi.org/10.1002/btpr.50
O’Mara, B, Gao, ZH, Kuruganti, M, et al. Impact of depth filtration on disulfide bond reduction during downstream processing of monoclonal antibodies from CHO cell cultures. Biotechnology and Bioengineering. 2019; 116: 1669– 1683. https://doi.org/10.1002/bit.26964
ABOUT THE UNIFUGE FAMILY
The UniFuge family provides low-shear separation, high-recovery performance, and fast processing times in three scalable models: UFMini, UniFuge Pilot, and U2K. With a variety of bowl sizes and flow rates ranging from 29 ml/min to 20 L/min, the UniFuge family of single-use separation systems offer both scalability and process efficiency in an aseptic closed system. The UniFuge Pilot was the industry pioneer in single-use centrifuge separation technologies. In 2021 CARR Biosystems released the UFMini to better support customers with a need for smaller scale processing using single-use technology.
ABOUT CARR BIOSYSTEMS
Founded in 1993 in Medfield, Massachusetts, CARR Centritech introduced the Powerfuge and continued to develop advanced separation systems for the Life Sciences and Specialty Chemical markets with the Viafuge, Centritech, and UniFuge models. In 2022, CARR Centritech transitioned to CARR Biosystems with continued emphasis on developing high-quality products to meet safety and quality standards throughout a variety of applications across the globe.