
Enhanced Cell Viability
- Low-shear separation to preserve cell health
- Efficient cell washing and buffer exchange to remove spent media or reagents
Streamline your cell therapy development and manufacturing with a scalable, low-shear platform designed to maintain cell viability. UniFuge® offers capabilities beyond cell harvesting, supporting gene editing, cryopreservation, and bioreactor expansion for consistent results from research through large-scale production.
The UniFuge platform uses tubular bowl technology for gentle cell processing, ideal for preserving cell viability. Automating key processes like cell separation and media exchange, UniFuge maximizes workflow efficiency for cell therapy applications. Its single-use, closed design minimizes contamination risks while streamlining critical unit operations to ensure reliable results.
Connect with an ExpertGene EditingGentle separation concentrates cells and exchanges media, preserving cell integrity before gene editing. | Cell BankingGentle separation concentrates cells and exchanges media, preserving cell integrity before gene editing. | Seed TrainScalable processing efficiently separates and concentrates cells, preparing them for transfer to larger bioreactors for further expansion. |
Expansion & DifferentiationMedia exchange and optional washing remove residual media while maintaining cell health for continued growth or differentiation. | Cell HarvestLow-shear separation gently concentrates cells, minimizing damage with optional washing to enhance purity. | FormulationCells are concentrated and media exchanged with formulation buffers, preparing them for cryopreservation with minimal loss and high viability. |



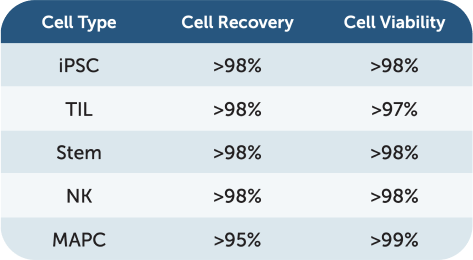
UniFuge delivers high cell viability and recovery, achieving over 98% recovery for iPSC, TIL, stem, and NK cells with over 99% viability. For MAPC cells, it ensures over 95% recovery and 99% viability.
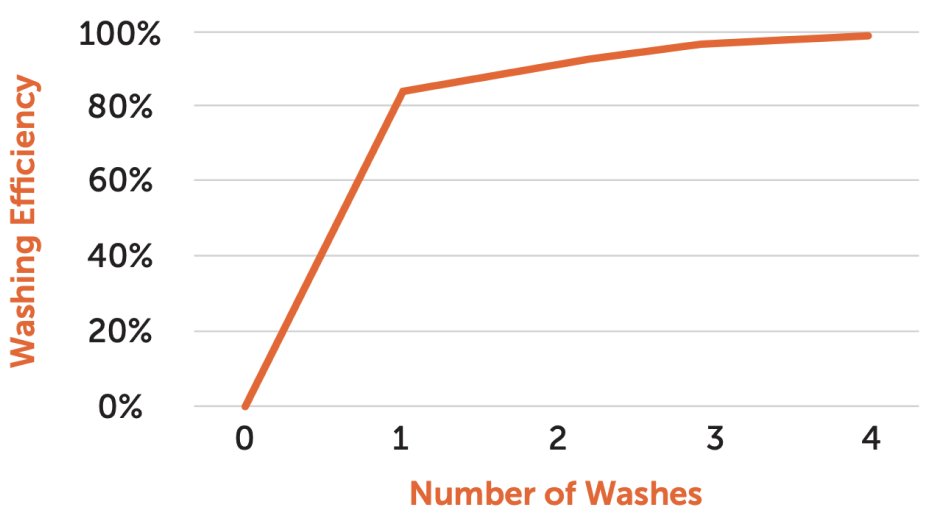
UniFuge demonstrates >99% washing efficiency after just two washes, effectively removing residual media and additives.
During cell therapy manufacturing, maintaining cell viability and recovery during harvesting is critical, but many available technologies fall short. Some provide high recovery but damage cells, while others preserve viability but underperform in recovery.
CARR Biosystems partnered with a customer to test the UniFuge Pilot and UniFuge Mini for cell harvesting in various cell types, including stem cells, NK cells, and MAPCs. Different g-forces (400-1200 x g) and flow rates (100-1,600 mL/min) were evaluated to optimize recovery efficiency and minimize viability reduction across multiple operating conditions.
The UniFuge platform delivered:

Get in touch with a sales representative, request support or download our free white paper.
Contact Us