Case Study: A joint investigation by PBS Biotech and Carr Biosystems
Abstract
Successful commercialization of pluripotent stem cell (PSC)–derived therapies requires robust, large‐scale cell processing operations - including expansion, differentiation, harvest, and concentration - that preserve critical cell attributes at every step. Although recent advances have enabled scalable PSC expansion, efficient downstream cell processing solutions for PSC aggregates remain underdeveloped. Preserving PSC aggregate properties is essential for bioreactor-to-bioreactor passaging in differentiation seed trains and for establishing high-quality PSC banks for downstream manufacturing. Closed, low-shear, automated processing technologies are therefore needed to safeguard cell viability, ensure reproducibility, and enable industrial scalability across all production stages.
In this work, the performance of advanced cell-processing technologies - specifically UniFuge® Cell Processing Platform and Vertical-Wheel® bioreactors - was assessed for scalable PSC aggregate manufacturing. Two experimental setups were implemented: (1) an open tube vs. closed tubular bowl centrifugation comparison to evaluate single-cell recovery and subsequent passage growth rates; and (2) the use of tubular bowl centrifugation during serial PSC expansion and harvest over multiple passages. Key metrics were rigorously quantified, including cell recovery efficiency, post-harvest proliferation rates, aggregate morphology, and pluripotency marker expression. Complementary process modeling was performed for batch volumes from 60 mL up to 80 L, relating batch size to processing time and throughput to confirm scalability without compromising cell health or recovery efficiency.
Integration of tubular bowl centrifugation with Vertical-Wheel® bioreactors yielded a reproducible, high-yield platform for PSC aggregate culture, supporting the industrial translation of PSC-derived therapies. Tubular bowl centrifugation matched conventional benchtop centrifugation methods in recovery and growth rates while limiting cell loss in waste streams to <1%. Across three serial passages, the integrated process consistently achieved >90% cell recovery and >95% post-harvest viability, with uniform aggregate formation, stable fold expansion, and displayed robust pluripotency marker expression. These results demonstrate the practicality of closed, low-shear, automated processing as a scalable and reliable solution for large-scale PSC production.
Keywords
Induced pluripotent stem cells (iPSCs); Cell Therapy; Scale-up; Cell recovery; Cell functionality; Tubular bowl centrifugation; Vertical-Wheel bioreactor; Automated processing; Industrial scalability; Allogeneic cell therapy
Introduction
Induced pluripotent stem cells (iPSCs) have revolutionized regenerative medicine by offering a versatile cell source capable of differentiating into a multitude of cell types. Their therapeutic potential has spurred significant research and development efforts. However, the translation of iPSC-based therapies from bench to bedside is hampered by challenges in scalable production of these shear sensitive cells. Processes used at critical production stages—such as cell expansion and differentiation, media exchange, harvest, and concentration—are prone to inducing cellular stress, particularly when transitioning from small-scale laboratory settings to industrial-scale manufacturing.
During scale-up, the physical forces involved, notably shear stress and mechanical agitation, can adversely affect cell health and compromise pluripotency. Traditional open centrifugation methods, while effective at laboratory scales, may result in inconsistent processing outcomes during development and are unable to be applied to larger volumes. In contrast, closed, single-use systems such as tubular bowl centrifuges offer the potential for reduced shear, minimized contamination risks, and improved reproducibility through each scale. Furthermore, by combining centrifugation with a bioreactor system, cells are gently handled and promptly returned to a consistent growth environment throughout expansion and differentiation. This coordinated approach maintains consistent cell conditions and enhances scalability throughout the scale-up process.
This study aims to address the critical need for scalable and reproducible iPSC production methods by evaluating advanced processing technologies. Specifically, we compare the performance of open versus closed centrifugation methods for iPSC recovery and growth maintenance and assess the efficacy of tubular bowl centrifugation in conjunction with Vertical-Wheel Bioreactors during serial cell expansion and harvest. Additionally, we share a process model that correlates processing parameters—including time, working volume, and throughput scalability—across bioreactor configurations enabling 60 mL to 80 L working volumes. Through this integrated approach, we seek to demonstrate that low-shear, automated processing systems can maintain high cell viability and pluripotency while supporting industrial-scale iPSC manufacturing.
Materials and Methods
Cell Line and Culture Conditions
The well-characterized iPSC line, TC1133, was used in this study. Prior to dissociation, cells were maintained in the PBS-3 Vertical-Wheel Bioreactor for 7 days using in-house culture protocols optimized for PSC expansion. Passaging was performed using large-scale in-vessel enzymatic dissociation and cell harvest protocols, and cultures were regularly monitored for morphology and pluripotency marker expression using flow cytometry.
Experimental Design
Study 1: Comparison of Open vs. Closed Centrifugation Methods
The first study was designed to compare conventional open conical tube centrifugation with closed, single-use, tubular bowl centrifugation.
Condition 1: Post-dissociation, iPSC cultures were subjected to a standardized benchtop centrifugation protocol at 500 x g for 5 minutes with acceleration and deceleration rates of 9 and 7 respectively.
Condition 2: Post dissociation, iPSC cultures were processed in the tubular bowl centrifugation system at 500 x g at a flowrate of 200 mL/min.
For both conditions, single-cell recovery was measured immediately post-centrifugation. Recovered cells were inoculated into matrigel-coated T-25 flasks and PBS-0.1 Vertical-Wheel Bioreactors for 2D and 3D growth studies. Growth rates were subsequently monitored over a 4 -day period in 2D and a 7-day period in 3D by cell counting and imaging analysis.
This setup allowed for a direct comparison of the recovery efficiency and short-term proliferation potential between the two centrifugation methods.
Study 2: Evaluation of Tubular Bowl Centrifugation in Serial Expansion
In the second study, iPSC cultures were serial passaged for a total of 4 passages in the PBS-3 Vertical-Wheel Bioreactor system. At each passage, the cells underwent the previously mentioned in-vessel dissociation and harvest protocol and were concentrated using the tubular bowl centrifugation system. This study enabled the assessment of the impact of large-scale serial passaging on aggregate morphology, cell proliferation rates, and maintenance of pluripotency marker expression. Key performance metrics included:
- Recovery Efficiency: Defined as the percentage of viable cells recovered relative to the initial cell count.
- Proliferation Rates: Determined by fold expansion during each passage.
- Aggregate Morphology: Assessed via phase-contrast microscopy.
- Pluripotency Marker Expression: Quantified by flow cytometry for markers including OCT4 and SOX2.
Process Modeling
A process model was constructed to correlate processing times, working volumes, and throughput scalability across various bioreactor scales. The model incorporated processing setpoints from bench-scale and large-scale experiments (100 mL and 3L) and extrapolated to larger volumes (up to 80 L). Key parameters included:
- Scaled flow rate, washing, and recovery parameters.
- Processing Time: time required for cell harvest and target concentration.
- Working Volume: total volume processed per run.
Analytical Techniques
- Flow Cytometry: Employed to assess the expression of pluripotency markers. Cells were stained with fluorescent antibodies against OCT4 and SOX2 and analyzed using the BD Accuri C6 Flow Cytometer
- Cell Counting: Viable cell counts were obtained using the Chemometec NucleoCounter NC-200 and Via-1 Cassettes
- Microscopy: Aggregate morphology was evaluated using the Nikon TS2-FL
Results
Performance Comparison: Open vs. Closed Centrifugation Methods
In Study 1, the performance of open conical tube centrifugation was compared with that of the closed, single-use, tubular bowl centrifugation system. The tubular bowl system demonstrated a recovery efficiency of 89.9%, closely matching the 93.3% observed with open centrifugation. Importantly, data indicated cells processed using both centrifugation methods experienced similar cell growth rates over the 7-day subsequent culture evaluation period in PBS-0.1.
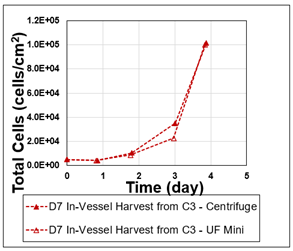
Figure 1: Post-dissociation cell growth on 2D substrate.
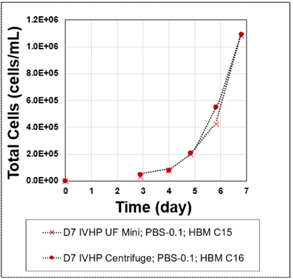
Figure 2: Post-dissociation cell growth in Vertical-Wheel bioreactor.
Aggregates formed successfully in both conditions on day 3 (Image 1, not representative of cell number on day 3).
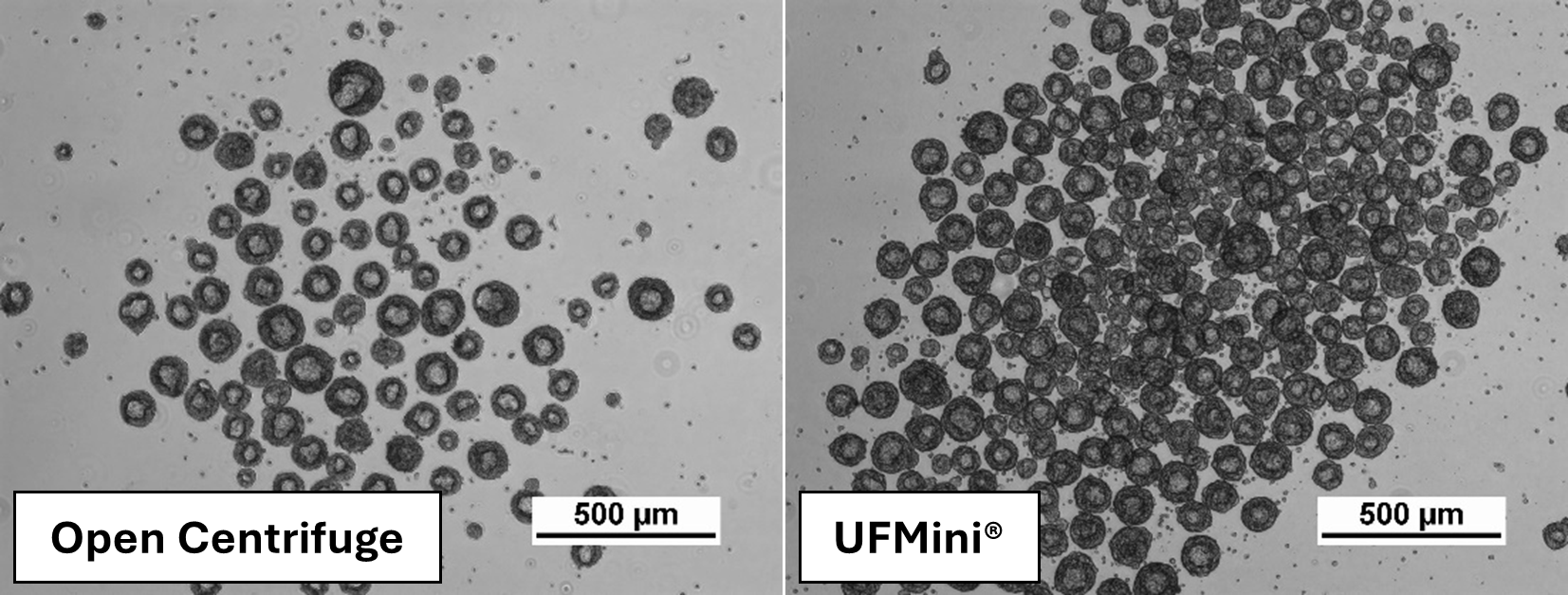
Image 1: Cell images comparing the aggregate morphology of the two different harvest methods on the same day. Aggregates formed successfully in both conditions on day 3.
Serial Expansion and Harvest Using Tubular Bowl Centrifugation
Study 2 investigated the effects of large-scale dissociation and harvest protocols and cell concentration using tubular bowl centrifugation on cell proliferation, aggregate formation, and pluripotency marker expression during serial passaging. Over multiple passages, cells consistently formed spherical and uniformly sized aggregates (Image 2).
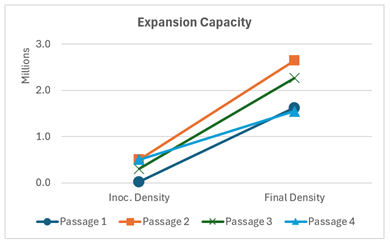
Figure 3: Study 2, the effect of passaging on cell proliferation measured in total cell count.
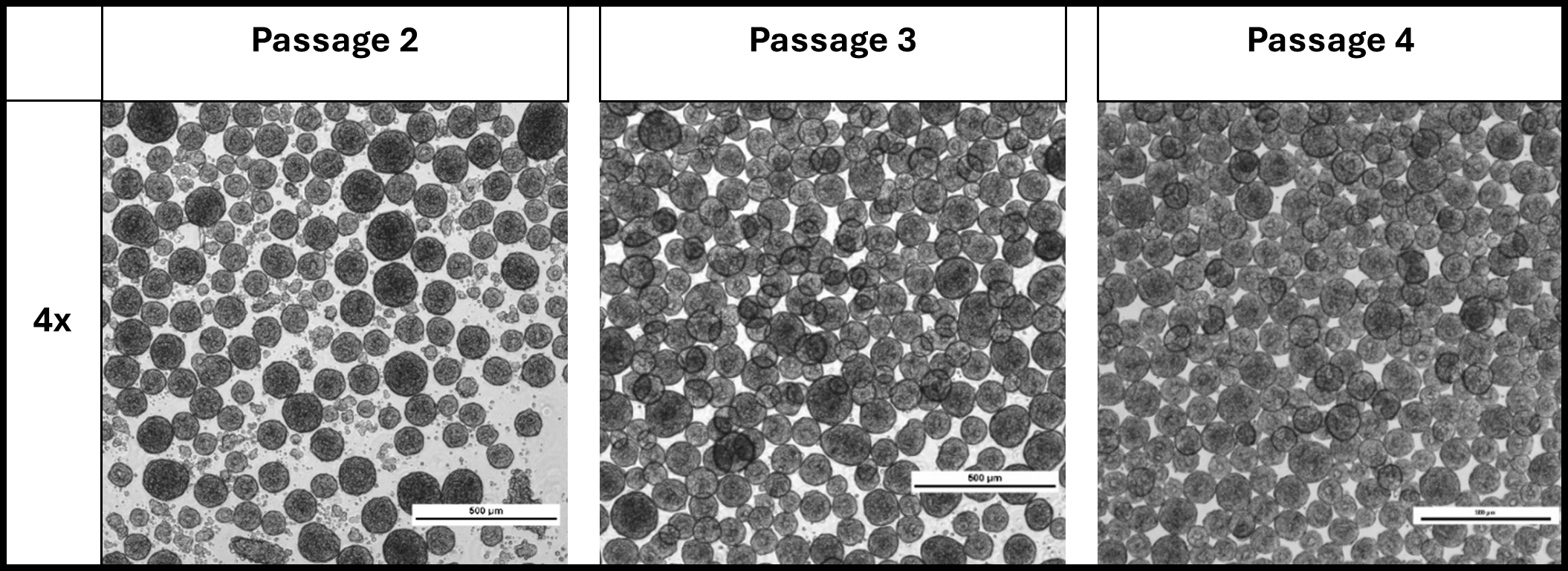
Image 2: Study 2 aggregate formation during subsequent passaging in the Vertical-Wheel bioreactor.
Flow cytometry analysis revealed robust expression of key pluripotency markers (OCT4, SOX2), with expression levels remaining unchanged from baseline through successive passages (Figure 3). These findings underscore the capability of the tubular bowl centrifugation system to support long-term maintenance of cell quality and pluripotency during serial expansion.
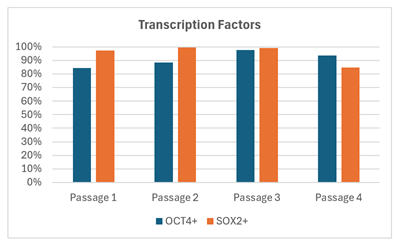
Figure 4: the effect of serial expansion using tubular bowl centrifugation measured by flow cytometry analysis of OCT4 and SOX2 pluripotency marker expression.
Process Model Insights on Scalability
The process model demonstrated processing times as cell culture volumes increase from 0.5L to 80L by leveraging observed performance at the bench scale to guide scalability decisions. By providing insights into process duration, the model illustrates the differences in scale-up and scale-out strategies. This predictive capability helps developers balance efficiency, cost, and quality, facilitating the transition from small-scale research to industrial-scale iPSC production.
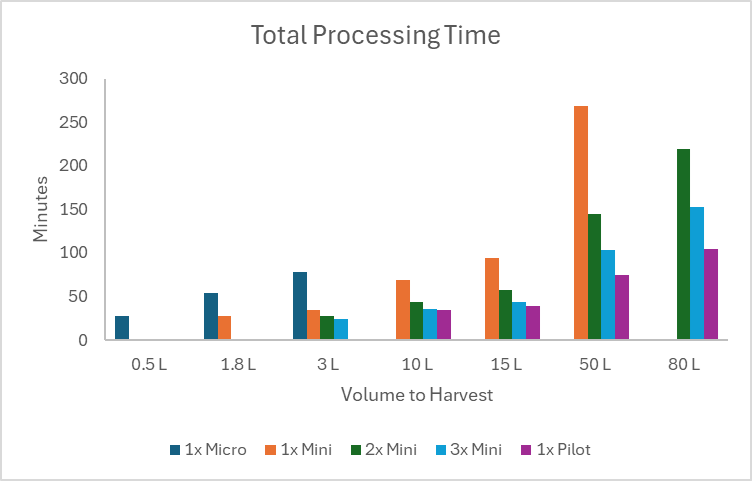
Figure 5: Process model projecting centrifugation duration across scale-up and scale-out strategies for iPSC production corresponding to 1.5x106 viable cell concentration.
Discussion
The data presented in this study underscores the potential of low-shear, automated processing technologies to address the scale-up challenges inherent in iPSC production. Traditional methods, while effective at laboratory scale, are open processes, increasing risk of contamination, are not scalable, and often increase process time resulting in stress that adversely affects cell health and quality. Our comparison between open benchtop centrifugation and closed, single-use tubular bowl centrifugation demonstrated that the latter maintains cell yield and cell quality comparable to conventional methods while enabling closed system processing and scalable options to minimize process time and simplify handling of large volumes.
The serial expansion study further illustrates the effectiveness of tubular bowl centrifugation in conjunction with a Vertical-Wheel Bioreactor. The maintenance of uniform iPSC aggregate formation, stable fold expansion, and high expression levels of pluripotency markers over successive passages indicates that the low-shear environment provided by the tubular bowl centrifugation system effectively minimizes cellular stress. This is particularly important given that prolonged exposure to shear forces and suboptimal processing conditions during scale-up can lead to irreversible changes in cell phenotype and loss of pluripotency.
The consistent pluripotency marker expression observed in these experiments are indicative of the combined system's ability to support the production of high-quality iPSCs suitable for clinical applications. The integration of automated, low-shear processing methods thus represents a significant advancement in iPSC manufacturing technology, offering a reproducible platform that can be seamlessly scaled from benchtop to industrial production.
Furthermore, the process model provides additional support for the scalability of this integrated system. By accurately correlating processing times, working volumes, and throughput scalability, the model serves as a valuable tool for anticipating the performance of the processing system in large-scale manufacturing settings. The consistency in recovery efficiency and cell viability across a wide range of volumes reinforces the system’s potential for industrial application, where reproducibility and scalability are critical.
It is important to note that while the current study provides compelling evidence for the advantages of tubular bowl centrifugation and Vertical-Wheel bioreactor integration, further research is warranted. Future studies should focus on the impact of functional differentiation potential of PSCs processed during media exchange conditions, and the economic analyses at manufacturing- scale will be essential to fully realize the industrial potential of these technologies.
Conclusion
The scalable production of PSCs is a critical requirement for the widespread adoption of cell-based therapies. This study demonstrates that advanced, low-shear, automated processing systems—specifically single-use tubular bowl centrifugation integrated with Vertical-Wheel bioreactor technology—offer a robust and reproducible platform for iPSC manufacturing. The data indicates that this approach yields recovery and proliferation rates comparable to conventional methods, while maintaining high cell viability and pluripotency over multiple passages. The process model further validates the system's scalability across working volumes ranging from 60 mL to 80 L. These findings provide a strong foundation for the industrialization of PSC-based therapies, paving the way for more efficient, closed and scalable production processes that preserve cell functionality and ensure therapeutic quality.
This comprehensive study highlights the integration of closed, low-shear and automated processing technologies as a viable solution to the challenges of scaling up iPSC production. By maintaining high cell viability and preserving pluripotency during critical production stages, the proposed system supports the reproducible manufacturing of iPSC-based therapies at an industrial scale, ultimately facilitating their clinical application.