This study presents data from a cell therapy developer that integrated the UniFuge® Micro into their hematopoietic differentiation workflow, with results shared with CARR Biosystems for publication.
Abstract
Induced pluripotent stem cells (iPSCs) are a versatile cell source for therapeutic applications, but their expansion and differentiation require careful handling to preserve viability and ensure consistent outcomes. Media exchange is a particularly critical step, where shear stress and open-system risks can compromise cell quality and reproducibility. To address these challenges, closed, low-shear technologies are needed to support GMP-compliant iPSC manufacturing.
In collaboration with an advanced therapy organization, this study evaluated the UniFuge® Micro (UFMicro) single-use centrifuge as an alternative to gravity sedimentation during multi-week iPSC aggregate expansion and differentiation into hematopoietic stem cells (HSCs). The UFMicro system was assessed for its impact on cell viability, biomarker expression, aggregate morphology, and final HSC yield across two independent runs.
Results showed that the UFMicro enabled gentle and efficient media exchange, supporting comparable or improved hematopoietic marker expression and HSC recovery relative to the control method. These findings validate the UFMicro as a practical solution for integrating automated, low-shear media exchange into scalable iPSC workflows, advancing the development of commercially viable cell therapy manufacturing platforms.
Introduction
Induced pluripotent stem cells (iPSCs) have emerged as a foundational technology in the field of cell therapy manufacturing. Generated by reprogramming somatic cells into a pluripotent state, iPSCs possess the dual capabilities of unlimited self-renewal and differentiation into virtually any cell type. This pluripotency enables the generation of diverse lineages, including cardiomyocytes, neural progenitors, and hematopoietic cells, making iPSCs a versatile cell source for both autologous and allogeneic cell therapies.
Despite their promise, the expansion and directed differentiation of iPSCs remain technically challenging. A major bottleneck is the need to maintain cell viability and morphology during sensitive processing steps, particularly during harvest and media exchange. To support successful differentiation into the desired cell type, it is essential to implement media exchange technologies that are gentle on the cell aggregates, volumetrically precise, and enable efficient processing times within defined time constraints. iPSCs are highly susceptible to shear stress, which can compromise viability, alter phenotype, and hinder differentiation outcomes. Additionally, batch-to-batch consistency in media exchange processing is critical for achieving repeatable differentiation results. Accurate delivery of signaling modulators during media exchange is necessary to guide proper lineage specification. Therefore, gentle, automated, and robust media exchange processes are vital to preserve cell quality and ensure consistent differentiation results.
Conventional methods for harvest and media exchange often expose cells to excessive shear forces, while also introducing operational inefficiencies and open-system risks. Gravity-based sedimentation, though low in shear, is a manual, open process that limits scalability, increases labor demands, and introduces batch-to-batch variability. These limitations underscore the need for closed, scalable, and low-shear technologies to support GMP-compliant iPSC manufacturing.
This study was conducted in collaboration with a leading provider of iPSC-based cell therapy development and manufacturing solutions. Their team integrated the UniFuge Micro (UFMicro) centrifuge within a hematopoietic differentiation workflow to evaluate its impact on media exchange efficiency and cell quality. This work reflects the client’s commitment to advancing scalable, GMP-compliant processes for iPSC-derived therapies.
In this evaluation, the collaborating organization assessed the UFMicro single-use centrifuge as an alternative to gravity sedimentation for media exchange during multi-day iPSC expansion, with a focus on supporting differentiation into hematopoietic stem cell (HSC) populations.
The adoption of single-use centrifugation platforms represents a significant opportunity to improve iPSC process control, scalability, and compliance. These technologies are increasingly being explored to streamline workflows and enable consistent, GMP-aligned cell therapy production.
Materials
- UFMicro Single-Use Centrifuge
- UFMicro 100 mL Single-Use Consumable
- 250 mL Stirred Bioreactor System
- Flow Cytometry Instrument and associated biomarker reagents
- Cell Culture Medium
Methods
Somatic cells obtained from human donors were reprogrammed into induced pluripotent stem cells (iPSCs) and subjected to a multi-week hematopoietic differentiation protocol. This process involved three key stages. First, iPSCs were cultured under conditions that promoted the formation of embryoid bodies (EBs) and cellular aggregates. Next, these aggregates underwent expansion while early induction toward hematopoietic lineages was initiated. Finally, the aggregates were dissociated to release differentiated single hematopoietic stem cells (HSCs), which were then collected during the terminal harvest.
Media exchanges were performed eight times throughout the multi-week protocol, as shown in Figure 1, to maintain optimal culture conditions for cell expansion and differentiation. Two parallel methods were employed for media exchange: the UFMicro single-use centrifuge system and gravity sedimentation control. The UFMicro centrifuge provided an automated, closed-system approach for cell concentration, media replacement, and cell return to the bioreactor. The gravity sedimentation method involved temporarily stopping bioreactor agitation to allow cellular settling, followed by removal of spent medium through the reactor dip tube and replenishment with fresh medium using the same pathway. The study was conducted in duplicate to evaluate reproducibility of the methods.
Cell and aggregate morphology, lineage, and viability were continuously monitored using microscopy and flow cytometry throughout the differentiation process. Midway through the process iPSC aggregate induction efficiency was assessed through analysis of lineage-specific marker expression. At the end of the process, HSC differentiation was evaluated by measuring established HSC identity markers, and final yield was quantified by determining the concentration of single HSCs per milliliter of culture medium.
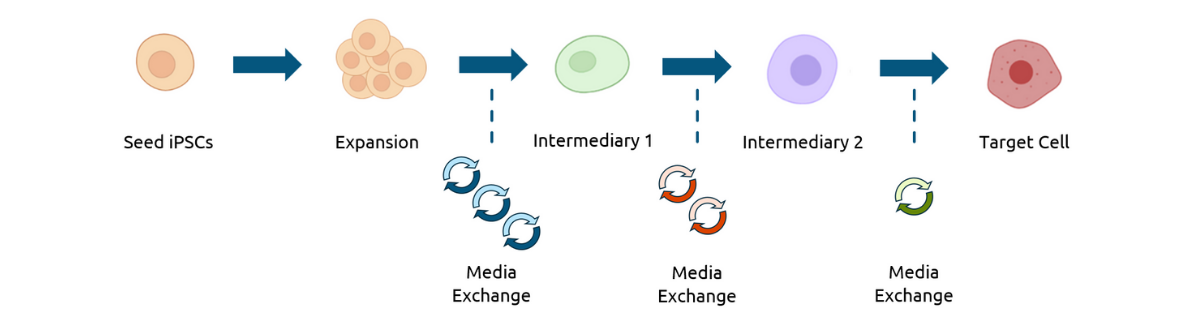
Figure 1: Process flow diagram for multi-week iPSC aggregate expansion and differentiation. The workflow includes eight total media exchanges, using three distinct exchange volumes and media compositions tailored to each stage of the differentiation process.
UFMicro Media Exchange Method
- Prefill bowl with media
- Feed 250 mL of cell culture, centrifuge
- Flush bowl with media
- Cell discharge
- Bowl chase
- Replenish with fresh media
Results
Midway through Run 1, the UFMicro condition showed 15% of iPSC aggregates positive for Biomarker Set 1 and 12% for Biomarker Set 2. The gravity sedimentation control showed 17% for Biomarker Set 1 and 13% for Biomarker Set 2. Midway through Run 2, the UFMicro condition showed 35% of iPSC aggregates positive for Biomarker Set 1 and 31% for Biomarker Set 2. The control condition showed 25% for Biomarker Set 1 and 23% for Biomarker Set 2 (Reference Figure 2).
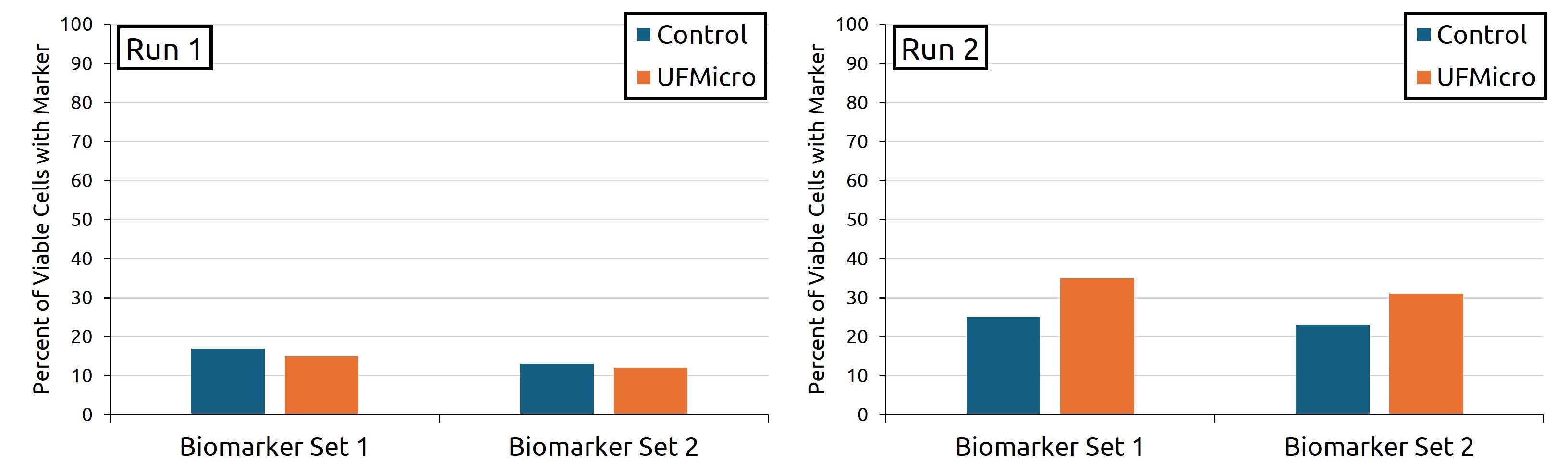
Figure 2: Two iPSC aggregate expansions (left: Run 1; right: Run 2) were evaluated midway through differentiation for the percentage of viable cells expressing two key biomarkers indicative of hematopoietic lineage commitment. Comparisons were made between the control media exchange method (gravity sedimentation in situ) and the UFMicro-based approach.
At the end of Run 1, the UFMicro condition showed 66.8% triple-positive hematopoietic stem cells (HSCs). The control condition showed 72.3%. At the end of Run 2, the UFMicro condition showed 89.3% triple-positive HSCs while the control condition showed 84.2% (Reference Figure 3).
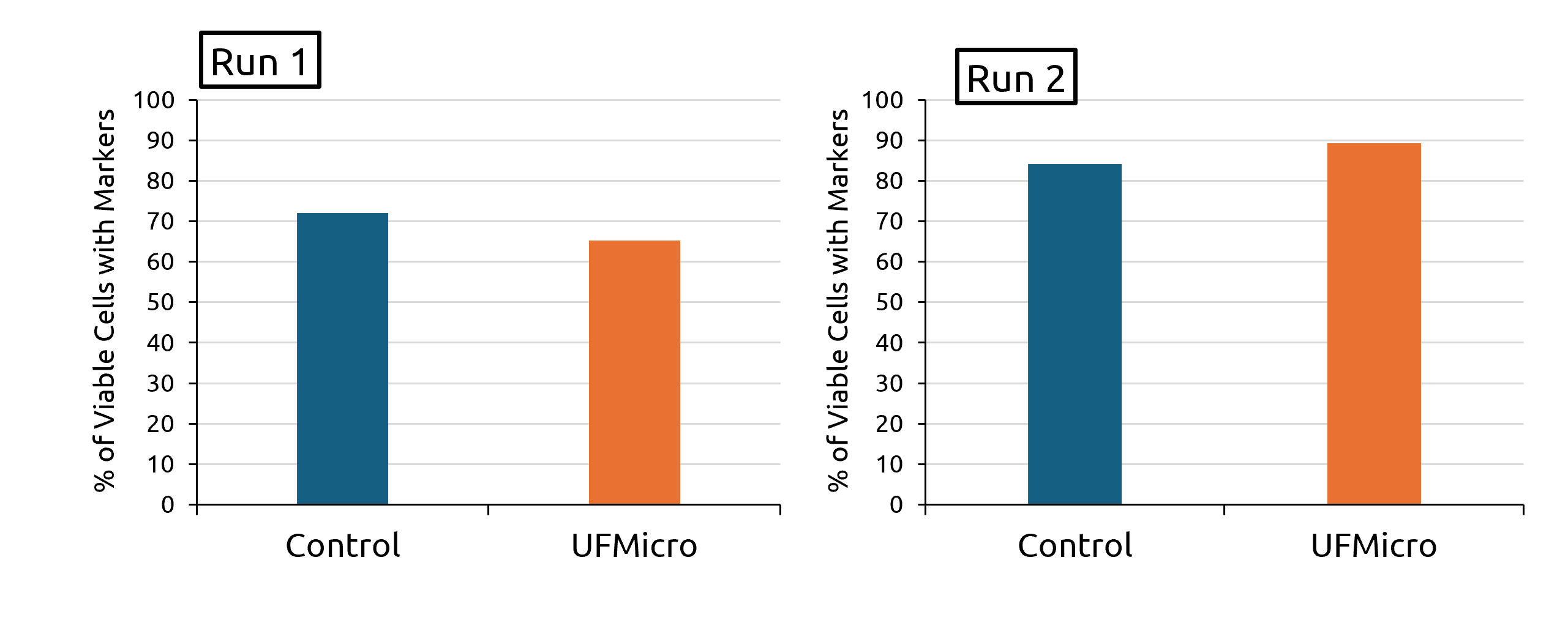
Figure 3: The percentage of viable cells expressing a critical biomarker indicative of HSC identity were evaluated at the end of the two protocols (left: Run, right: Run 2). Comparisons were made between the standard media exchange method (gravity sedimentation in situ) and the UFMicro-based approach.
At the end of Run 1, the HSC single-cell concentration in the UFMicro condition was 103% relative to its control, which was normalized to 100%. At the end of Run 2, the UFMicro condition reached 107% of its respective normalized control value (Reference Figure 4).
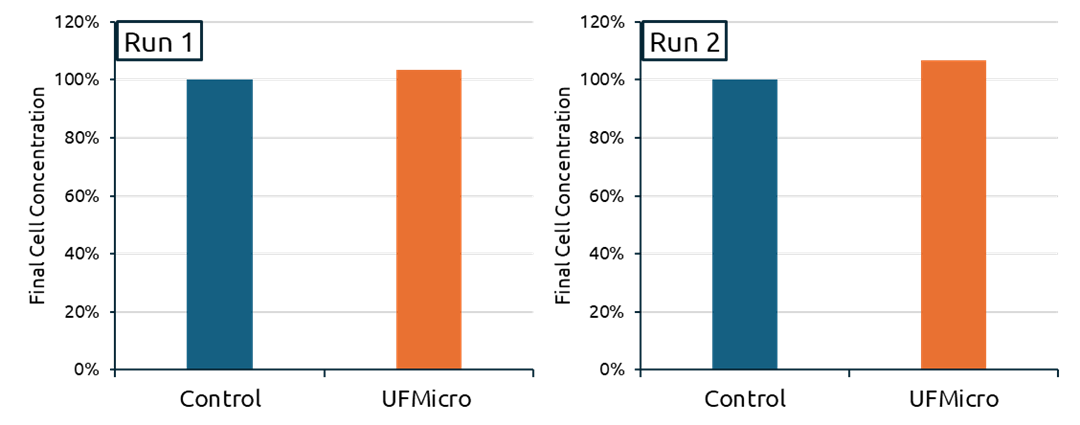
Figure 4: The concentration of released single cells expressing HSC identity were evaluated at the end of each protocol (left: Run 1, right: Run 2). Data was normalized to the control condition for each run to enable direct comparison of HSC yield and release efficiency between the standard media exchange method (gravity sedimentation in situ) and the UFMicro-based approach.
Morphology remained comparable between the UFMicro and control conditions throughout the process. iPSC aggregate size, shape, and quantity appeared similar at each stage, consistent with the observed biomarker profiles and final cell yields.
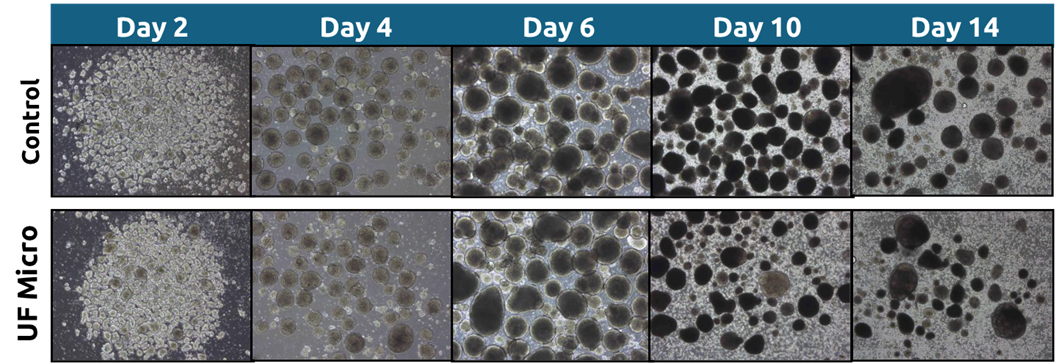
Figure 5: Cell morphology results from Run 1 were recorded on days 2, 4, 6, 10, and 14 of the protocol. Comparisons were made between the standard media exchange method (gravity sedimentation in situ) and the UFMicro-based approach.
Discussion
In Run 1, midway point iPSC aggregate marker expression in the UFMicro condition was comparable to the gravity sedimentation control, while in Run 2, the UFMicro condition showed enhanced expression. These results suggest that the UFMicro enabled gentle and effective media exchange in the beginning of the process, supporting early hematopoietic lineage commitment at levels equal to or exceeding those achieved with control method.
By the end of the protocols, both runs demonstrated that HSC identity marker expression in the UFMicro conditions remained consistent with the control, indicating that the system’s media exchange and handling processes effectively supported iPSC differentiation into hematopoietic stem cells. Additionally, single-cell HSC concentrations were similar across conditions, suggesting that the UFMicro facilitated the release and recovery of HSCs at levels comparable to the control.
These findings are particularly relevant to the development and manufacturing of iPSC-based therapies, where maintaining aggregate integrity is critical for consistent and high-quality differentiation outcomes. Given the sensitivity of iPSC aggregates to mechanical stress, which can compromise morphology and downstream differentiation. The ability of the UFMicro system to maintain aggregate integrity throughout the protocol comparable to the gravity sedimentation method underscores its value in processing fragile cell types.
Collectively, the results validate the UFMicro as a practical and effective media exchange solution for shear-sensitive cell cultures. Its ability to support robust differentiation while streamlining processing positions it as a valuable tool for advancing iPSC-based therapies toward scalable, commercially viable manufacturing platforms.
Conclusion
As iPSC-derived therapies gain momentum and move toward commercialization, the need for gentle, closed-system processing technologies becomes increasingly critical to preserve cell integrity and ensure consistent differentiation outcomes. This study demonstrates that the UFMicro single-use centrifuge enables efficient media exchange while maintaining aggregate morphology and supporting robust hematopoietic differentiation.
The system’s low-shear, automated design reduces the risks associated with open, manual methods and aligns with GMP manufacturing standards. By delivering consistent performance across extended protocols, the UFMicro addresses key bottlenecks in iPSC processing, particularly in media exchange and aggregate handling.
These findings position the UFMicro as a practical solution for integrating gentle cell handling into scalable workflows, offering the cell therapy industry a path toward more efficient, standardized, and commercially viable manufacturing platforms.